PIPELINE
THE PROBLEM
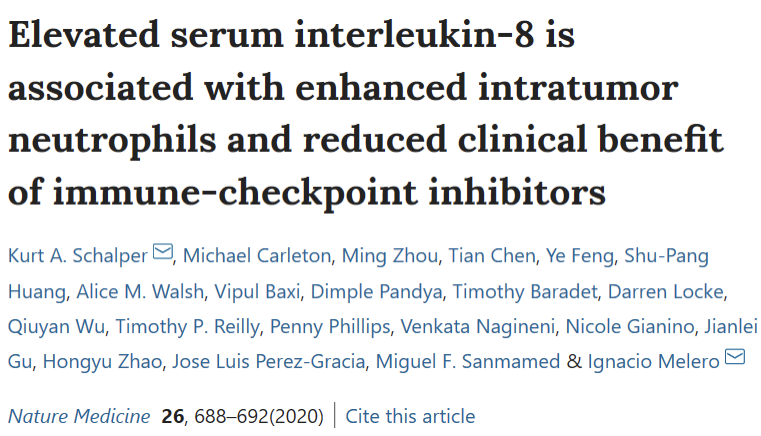
IL-1 Effects:
“Chronic inflammation mediated by IL-1 signaling leads to tumor immuno-suppression by myeloid cells (MDSCs)”
- Driving chronic non-resolving inflammation
- Tumor angiogenesis
- Activation of the IL-17 pathway,
- Induction of myeloid-derived suppressor cells (MDSC)
- IL-1 promotes recruitment of myeloid cells in tumors and their tumor promoting function
- IL-1β neutralization leads to differentiation of MDSCs into M1 anti-tumor macrophages, and it prevents the differentiation of inflammatory monocytes into suppressive macrophages.
- Macrophage recruitment
- Invasion
- Metastasis (EMT)
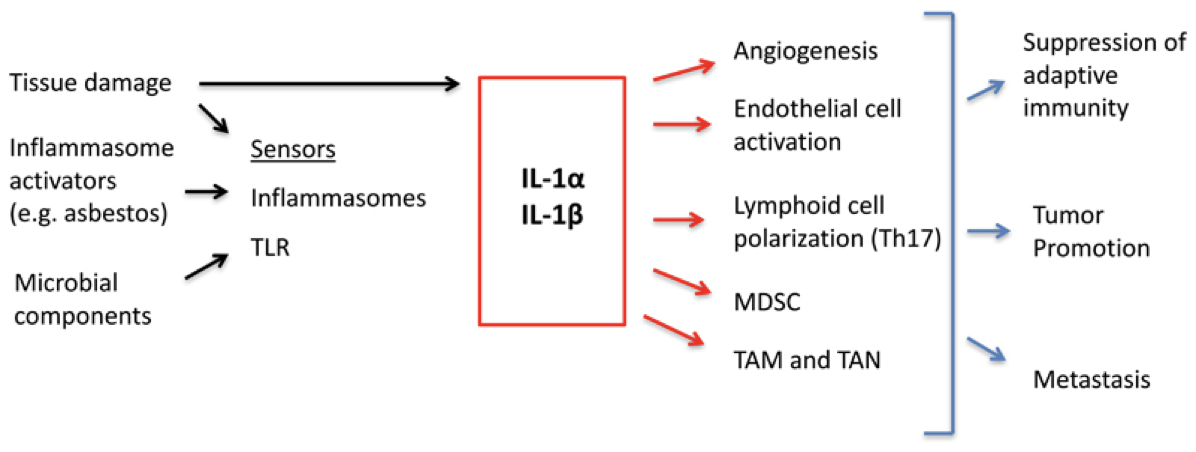
IL-1α and IL-1β are both important in cancer development

- IL-1α is constitutively expressed by mesenchymal and epithelial cells and is released as an alarmin to signal cellular damage/necrosis or as a signal of DNA damage
- IL-1β is not constitutively expressed, but is upregulated, primarily in monocytes and macrophages, following NFκB activation
- IL-1 initiates a signaling cascade which results in NFκB-mediated transcription, Cox-2 induction and production of pro-inflammatory mediators such as IL-6, IL-8 and prostaglandin E2
- Endogenous regulation of signaling occurs via tight control of IL-1α and IL-1β activation and release as well as production of the IL-1 receptor antagonist (IL-1RA)
In the tumor: Interleukin-1 release from tumor cells and macrophages
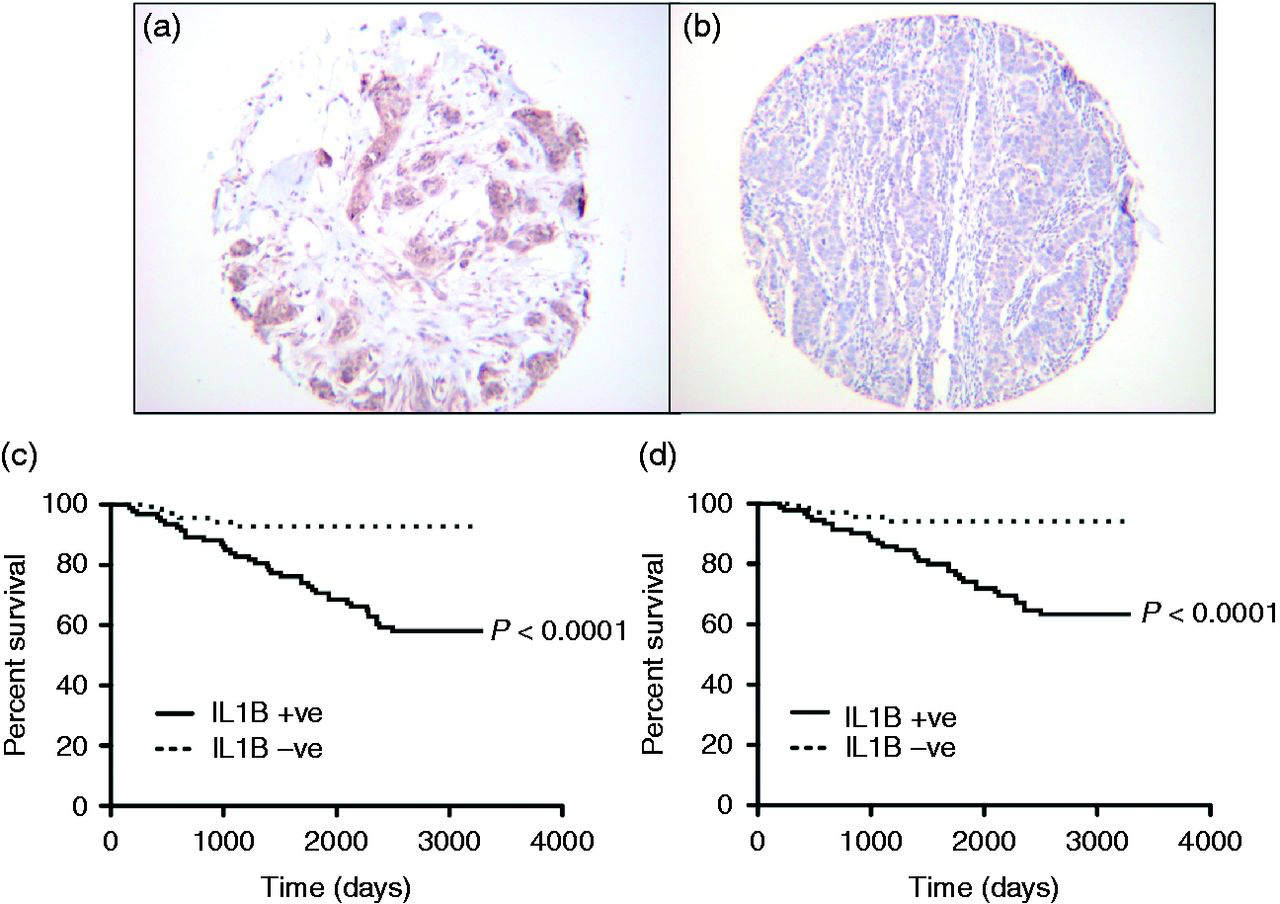
High IL-1 expression in cancer confers poor prognosis
Overexpression of interleukin-1β induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice
Read further: Tu et al. Cancer Cell. 2008 Nov 4;14(5):408-19.
- Polymorphisms of interleukin-1β (IL-1β) are associated with an increased risk of developing solid malignancies
- Stomach-specific expression of human IL-1β in transgenic mice leads to spontaneous gastric inflammation and cancer that correlates with early recruitment of myeloid-derived suppressor cells (MDSCs)
- IL-1β activates MDSCs ina vitro and in vivo through an IL-1RI/NF-kappaB pathway
- IL-1β transgenic mice deficient in T and B lymphocytes develop gastric dysplasia accompanied by a marked increase in MDSCs in the stomach
- Antagonism of IL-1 receptor signaling inhibits the development of gastric preneoplasia and suppresses MDSC mobilization
- These results demonstrate that pathologic elevation of a single proinflammatory cytokine may be sufficient to induce neoplasia and provide a direct link between IL-1β, MDSCs, and carcinogenesis
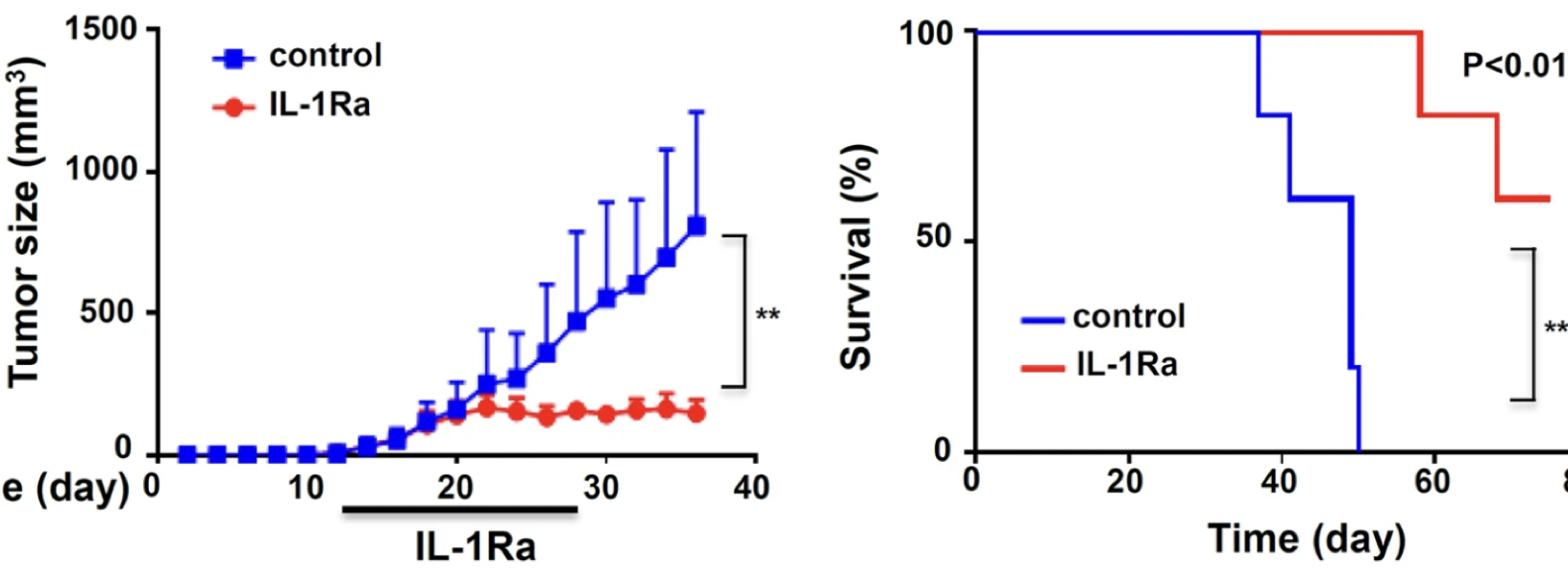
IL-1R blockade inhibits human breast cancer growth in an orthotopic xenograft model
- Model: Breast cancer cell line MBA-231
- Dosing: IL-1Ra 5mg/kg every 2 days
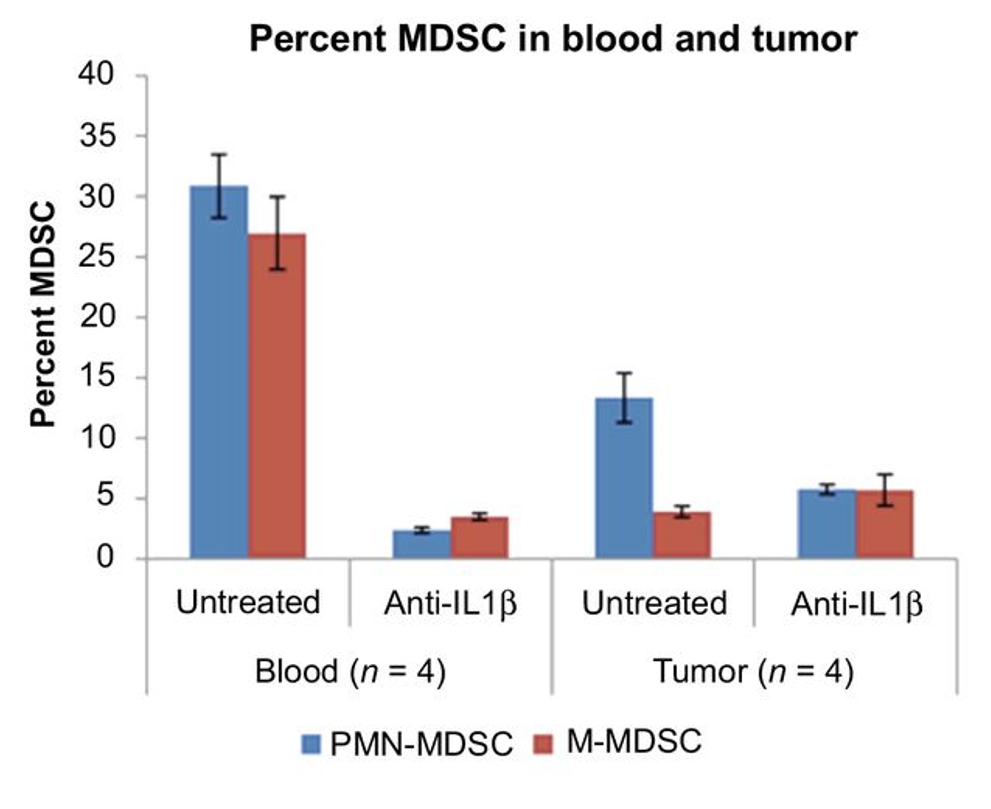
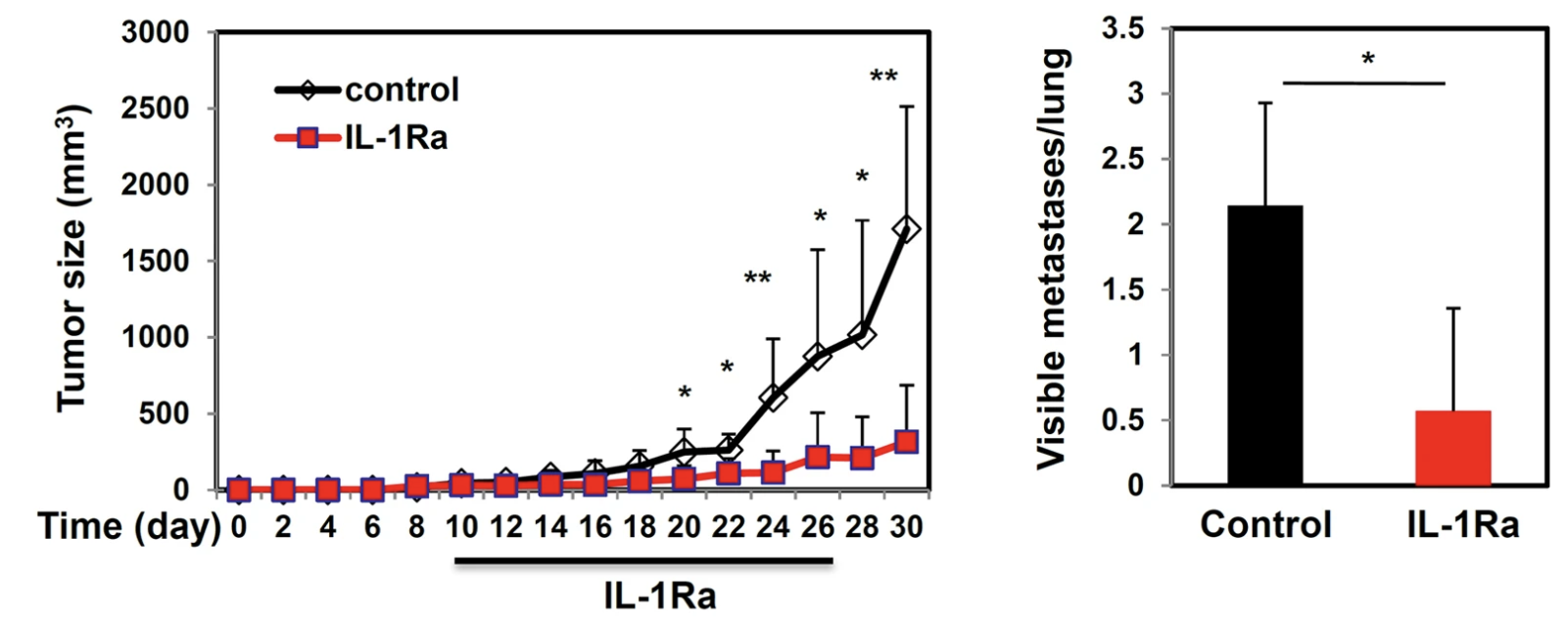
mIL1Ra treatment reduces GR1+ (G-MDSC) cells in the tumor, tumor growth and metastasis
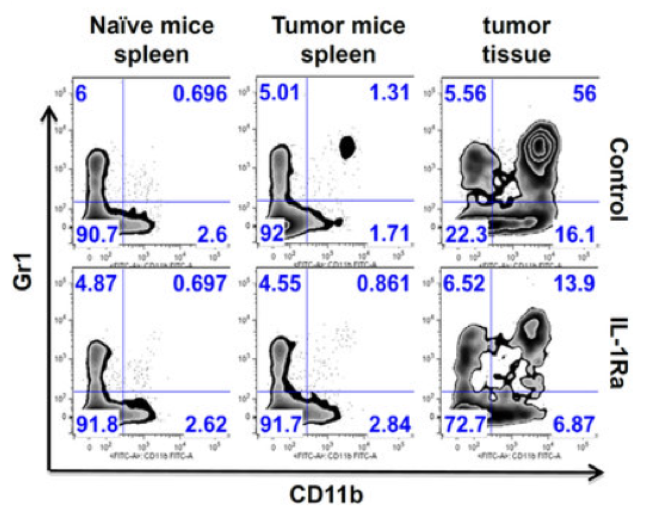
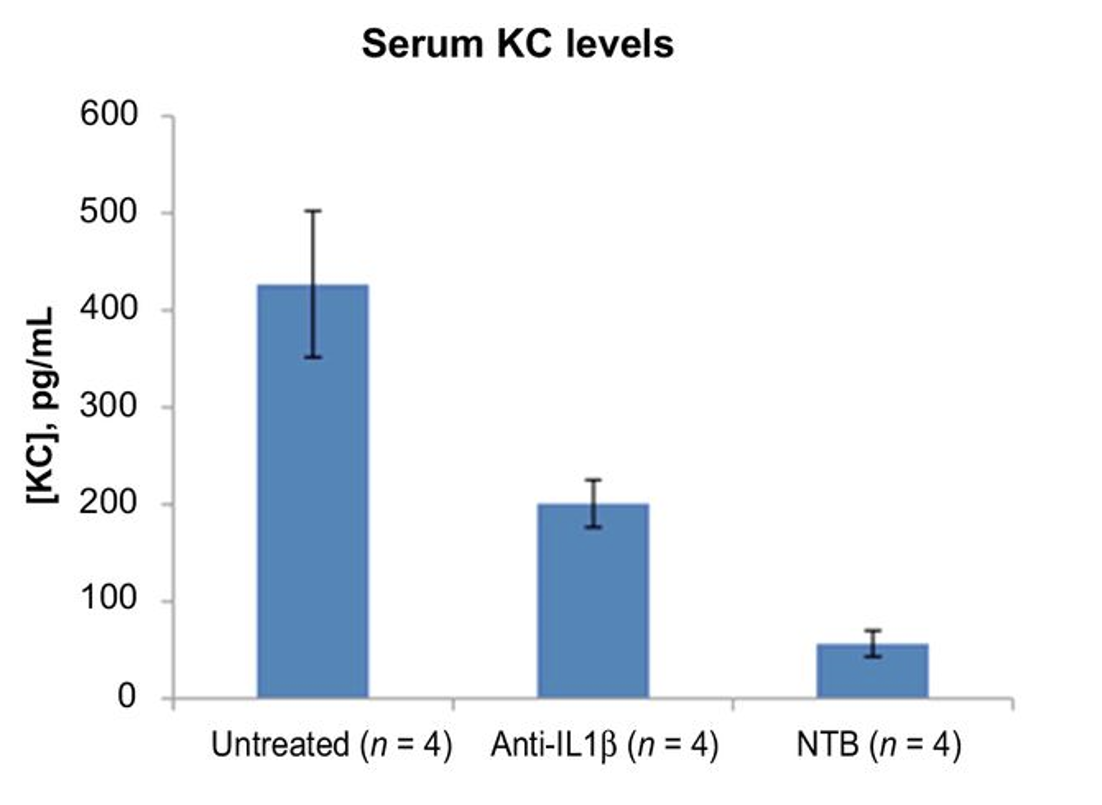
IL-1β mAb reduces G-MDSC and KC (IL-8) in mouse kidney cancer (RCC) model
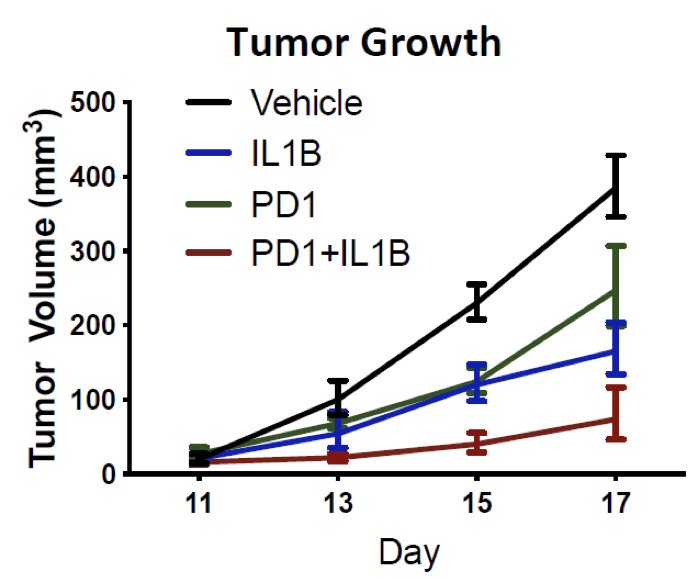
Combination of anti–IL-1β with anti–PD-1 or cabozantinib treatment improves the outcome of RCC in mice
- In a mouse model of RCC (RENCA), treatment with an anti-IL-1β antibody resulted in a significant decrease in tumor growth from day 10 to 18 compared to vehicle or anti-PD-1
- Combination treatment anti-IL-1β/anti-PD-1 or anti-IL-1β/cabozantinib potentiated the monotherapy effects
- anti-IL-1β therapy decreased the infiltration of granulocytic MDSCs (Gr-MDSC) (p=0.014) and increased M1-like TAM
- Based on these result the group at Columbia is conducting a clinical trial of neoadjuvant anti-IL-1β and anti-PD-1 in patients with high-risk RCC (NCT04028245)
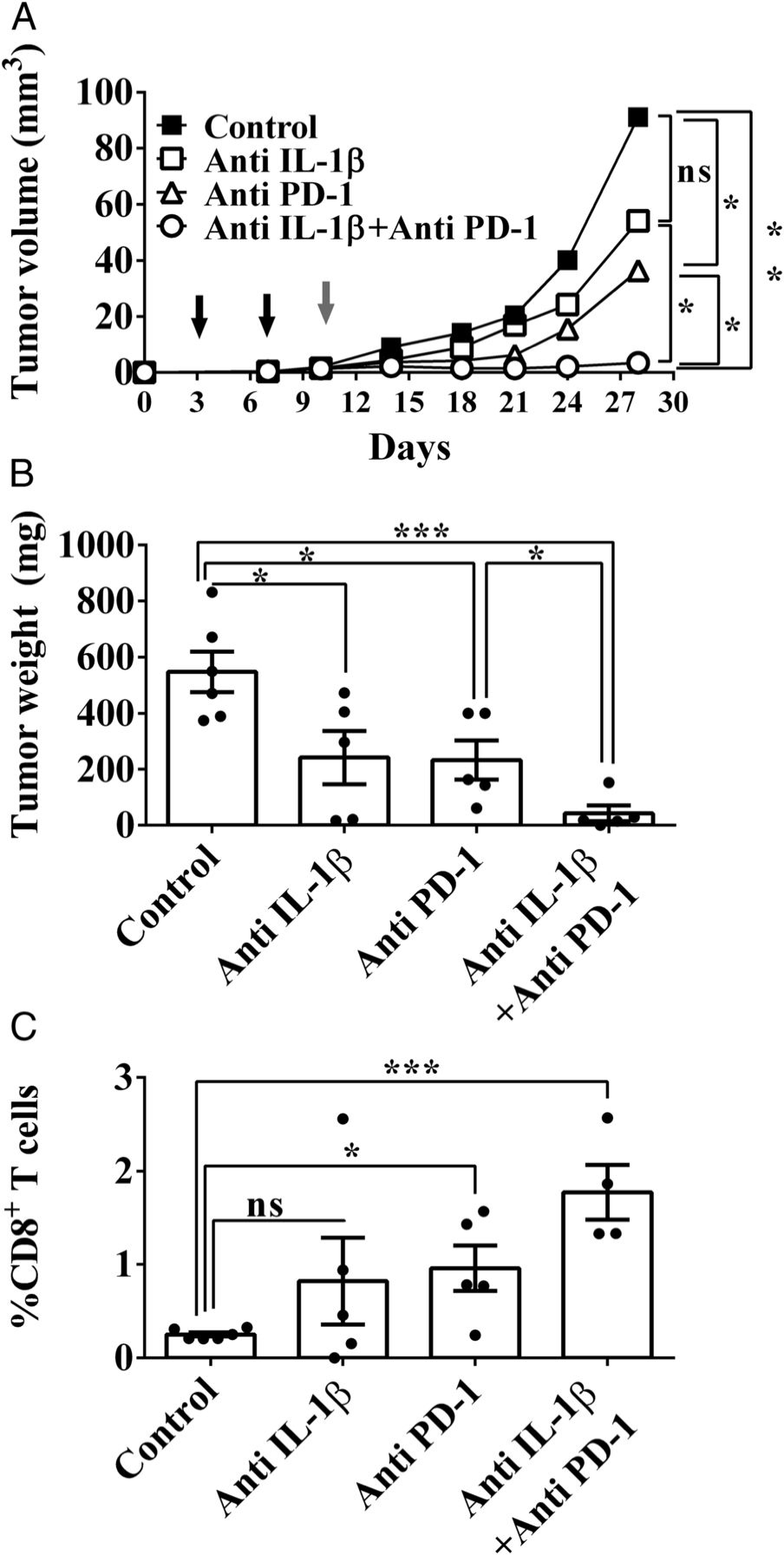
Combination of anti–IL-1β with anti–PD-1 treatment improves the outcome of 4T1 mammary carcinoma in mice
- A recent study shows that the combination of anti–IL-1β with anti–PD-1 treatment improves the outcome of 4T1 mammary carcinoma in mice
- Each modality on its own has a modest effect on tumor growth in this model, but when combined there appears to be a synergistic effect leading to near-complete inhibition of tumor growth for up to 30 days (as long as the experiment)
- IL-1 inhibition led to changes in the TME: lower levels of monocytes recruited into the tumor, leading to more CD8+ T cells
- “These data define microenvironmental IL-1β as a master cytokine in tumor progression”
THE SOLUTION
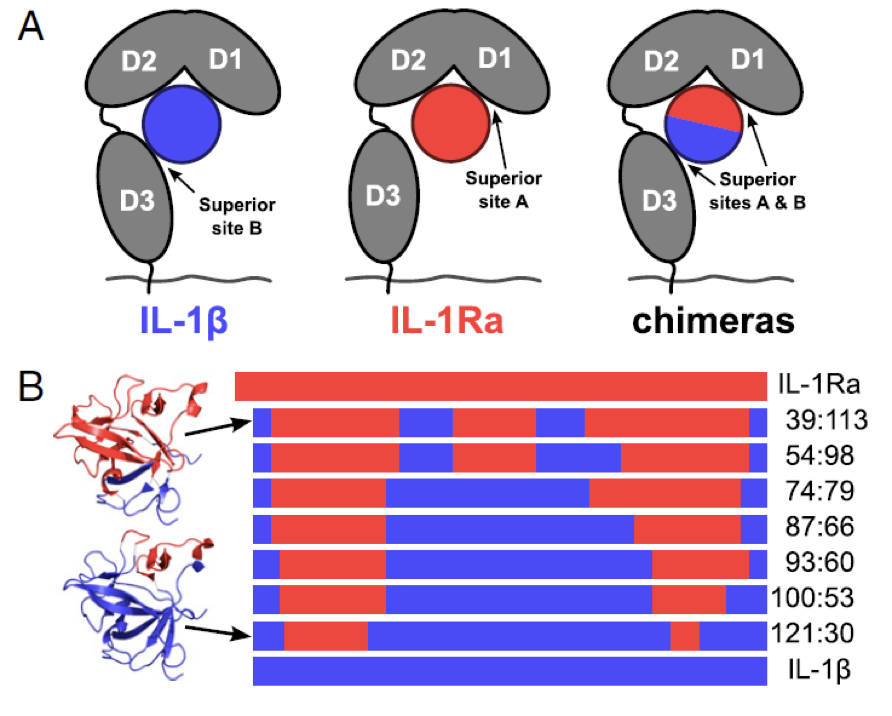
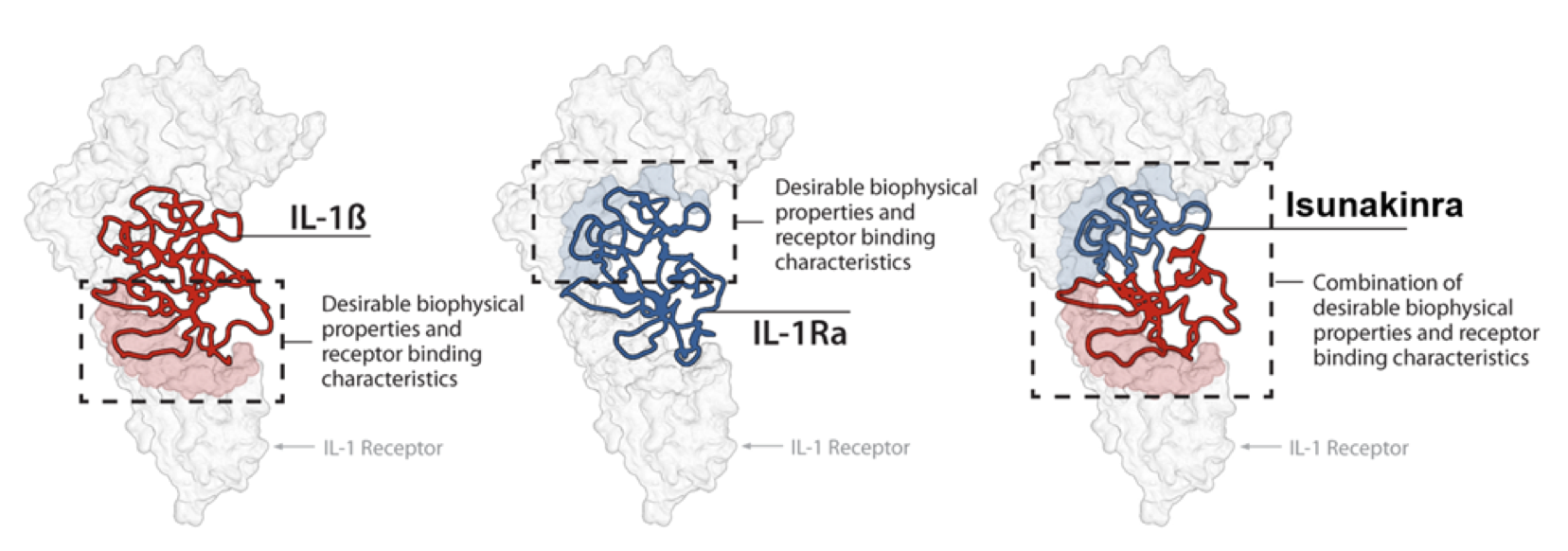
Isunakinra – a very potent IL-1 blocker
- Isunakinra (EBI-005) was selected from a range of chimeric proteins based on the sequences of IL1Ra and IL-1β
- Both cytokines bind to separate parts of the IL1 receptor (IL1R1)
- Isunakinra was selected to show:
- High potency (up to 20 x IL-1Ra)
- No agonistic effect
- Thermal stability
Isunakinra binds IL1R1 with high affinity and blocks the binding of the natural agonists: IL1α and IL1β
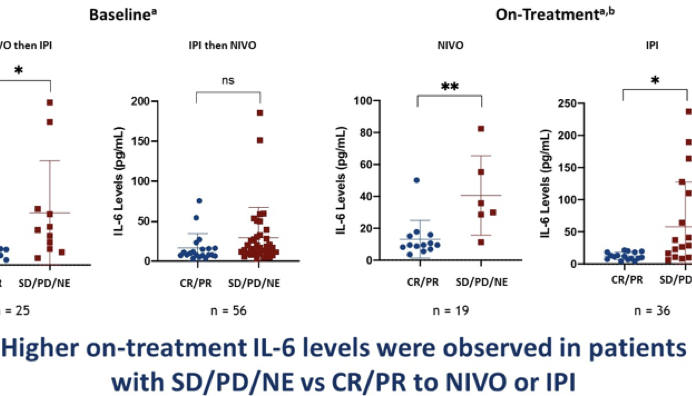
“CRP, IL-6 and IL-8 are prognostic factors for checkpoint inhibition”
- In Buzzard’s phase I/II trial we will use IL-6, IL-8 and hsCRP as pharmacodynamic biomarkers of IL-1 inhibition. IL-6 and IL-8 are downstream of IL-1 and CRP is downstream of IL-6.
- A recent study of IL-6 and CRP levels in melanoma patients on checkpoint blockade – showed that high IL-6/CRP correlated with poor response and poor prognosis:
- “High levels of CRP and IL-6 at baseline were associated with a poor response and shorter survival after nivolumab alone, and CRP with ipilimumab alone or the combination of both drugs. High levels of CRP were also associated with shorter overall survival in the randomized CheckMate 066 study of chemotherapy compared to nivolumab. CRP and IL-6 are prognostic factors for checkpoint inhibition.”
Isunakinra – is being tested in a clinical phase I/II trial in solid tumor patients – combining with a PD-1 inhibitor
- Buzzard is conducting a phase I/IIa trial in solid tumor patients Isunakinra taken as a daily subcutaneous injection (patient self-injection)
- Objectives:
- Establish safety and PK of isunakinra in patients at different doses
- Dose selection based on safety and effect on PD markers: CRP and IL-6 in plasma
- Establish safety and efficacy on biomarkers in combination with a PD-1/PD-L1 inhibitor
- Biomarkers include cytokines, Gr-MDSCs, CD8+ T cells and other leukocyte subsets in tumor biopsies, Circulating tumor cells (CTCs)
- Study to be conducted under an IND in collaboration with dr Carlos Becerra at Baylor University, Dallas, TX
- Enrollment is ongoing
- See study description at: https://clinicaltrials.gov/ct2/show/NCT04121442